Services
Full solution provider for mammalian cell culture
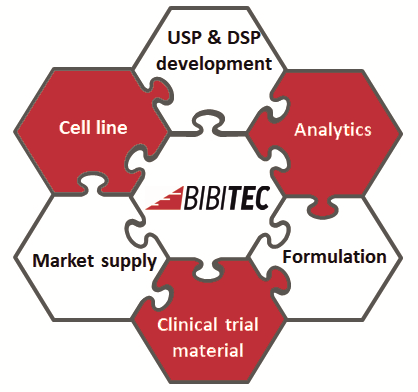
BIBITEC offers a full service for process development and GMP manufacturing of biopharmaceutical APIs for clinical trials up to phase III. We are specialized in the production of:
- Non-recombinant (native) proteins
- Monoclonal antibodies (mAb)
- Antibody fragments (Fab)
- Other recombinant proteins (e. g. enzymes, hormones)
In cooperation with Nordmark Biotech, we can accompany your process from cell line development to market supply.
Capacities
Our state-of-the-art production facilities allows for the following production capacities:
- Lab scale (up to 2 L) – for development
- Pilot scale (up to 200 L) – for clinical trials
- Production scale (up to 2.000 L) – for continuous market supply